Metals and Non-metals Worksheet For Class 8
Dive into the fascinating world of elements with our comprehensive Metals and Non-metals Worksheet for Class 8! Whether you're a budding chemist or just curious about the materials that make up the world around us, this worksheet is tailored specifically for your educational journey. Through engaging multiple-choice questions (MCQs) and detailed notes, you’ll explore the essential characteristics, uses, and properties that distinguish metals from non-metals. Perfect for self-study or as a classroom resource, this worksheet is designed to enhance your understanding of basic chemistry concepts covered in the curriculum.
Discover why metals like iron and copper are pivotal in our daily lives and how non-metals such as sulfur and carbon play crucial roles in various industries. Our Class 8 Metals and Non-metals Worksheet is not just a learning tool but a gateway to sparking interest in the elemental constituents of the universe. Each question in the worksheet challenges you to think and apply your knowledge, making learning a dynamic and interactive experience.
Prepare to ace your exams and deepen your understanding of these fundamental elements with our meticulously crafted Metals and Non-metals class 8 MCQ sheet. Whether it's the reactive series of metals, the conductivity of various elements, or the intriguing physical and chemical properties of non-metals, this resource is your go-to guide for all.
Enhance your learning further with our Metals and Non Metals Class 8 Notes PDF, a downloadable resource that complements the MCQ worksheet. It provides detailed explanations and insights, making complex concepts easier to grasp. This combination of practical questions and comprehensive notes ensures you have a well-rounded grasp of the topic, empowering you with the knowledge to excel in your studies and beyond.
Start your journey into the world of chemistry with confidence. Grab your copy of the Metals and Non-metals Worksheet for Class 8 today and begin exploring the intricate balance of elements that shape our world!
Metals and non-metals class 8
In Class 8, the chapter on Metals and Non-metals introduces students to the fascinating world of elements, dividing them into two primary categories based on their physical and chemical properties. This fundamental distinction lays the groundwork for understanding the periodic table and the role of these elements in various applications. Students learn about the conductivity, malleability, and reactivity of metals, contrasting these with the brittleness and insulating properties of non-metals. Through interactive experiments, such as testing the reactions of metals with water and acids, learners gain hands-on experience. This chapter not only enriches their knowledge of chemistry but also emphasizes the practical significance of metals and non-metals in daily life, from the construction of buildings to the electronics that power our world.
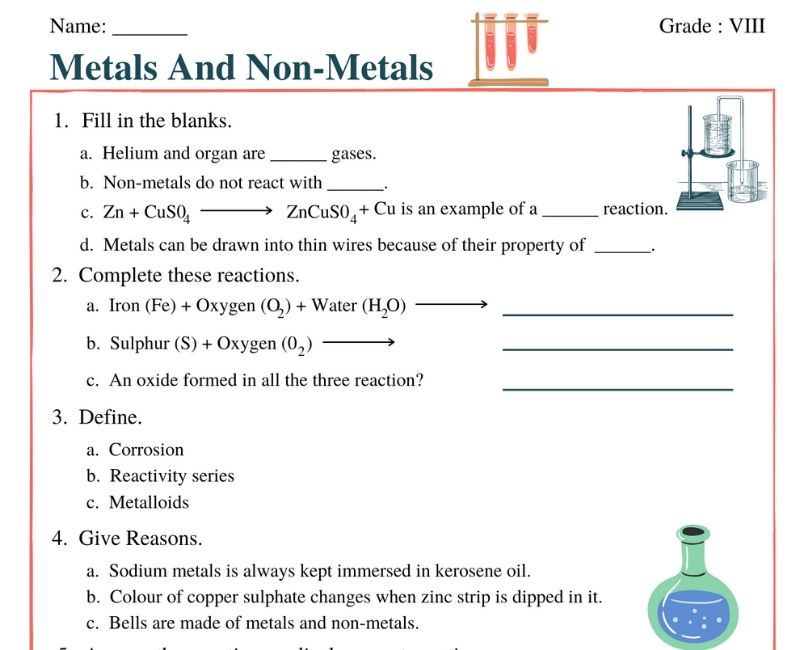
Metals and Non-metals Class 8 Worksheet
The Metals and Non-metals Class 8 Worksheet is a thoughtfully designed resource aimed at helping students distinguish between metals and non-metals, two of the primary categories of elements. These worksheets challenge students with exercises that include identifying, classifying, and understanding the unique properties and uses of metals and non-metals. Through engaging activities, learners can explore the practical applications of elements in real life, from the metallic sheen of copper to the non-metallic character of sulfur. This worksheet serves as an excellent tool for reinforcing concepts taught in class and encouraging independent research.
Metals and Non-metals Class 8 MCQ
Metals and Non-metals Class 8 MCQ sheets are a fantastic way for students to test their understanding of the topic in a quick and engaging manner. Multiple-choice questions cover a wide range of topics, from basic properties and examples of metals and non-metals to their reactions with other substances. By addressing a variety of scenarios, these MCQs ensure that students are not only recalling information but also applying their knowledge to different contexts. This approach aids in solidifying their grasp of the subject matter, making it an invaluable resource for exam preparation.
Metals and Non-metals Class 8 Notes PDF
The Metals and Non-metals Class 8 Notes PDF is a comprehensive document that outlines everything students need to know about metals and non-metals. From detailed explanations of their physical and chemical properties to their reactivity with other elements, these notes are meticulously organized to facilitate easy learning. Including illustrations and real-world examples, the PDF serves as a quick reference guide for revising key concepts and ensures students have access to reliable material to support their studies, anytime and anywhere.
Reaction of Metals and Non-metals with Acids Class 8
Understanding the Reaction of Metals and Non-metals with Acids is a crucial part of the Class 8 science curriculum. This section delves into how metals and non-metals behave differently when they come into contact with acids. Metals typically react with acids to produce hydrogen gas and a salt, an exciting demonstration of chemical change. In contrast, non-metals' reaction with acids is less pronounced, showcasing their distinct properties. Recognizing these reactions not only reinforces the theoretical distinction between metals and non-metals but also emphasizes the importance of safety and precision in chemical experiments.
Class 8 Metals and Nonmetals Extra Questions
Class 8 Metals and Nonmetals Extra Questions are an essential resource for students seeking to deepen their understanding of the subject. These questions go beyond the standard curriculum, challenging learners to think critically about the properties, uses, and significance of metals and non-metals in our world. From exploring advanced applications of these elements to analyzing their environmental impact, these extra questions encourage students to connect classroom knowledge with real-world scenarios. Providing a broader scope of learning, they are an excellent way for students to engage more deeply with the material and prepare for higher-level science courses.