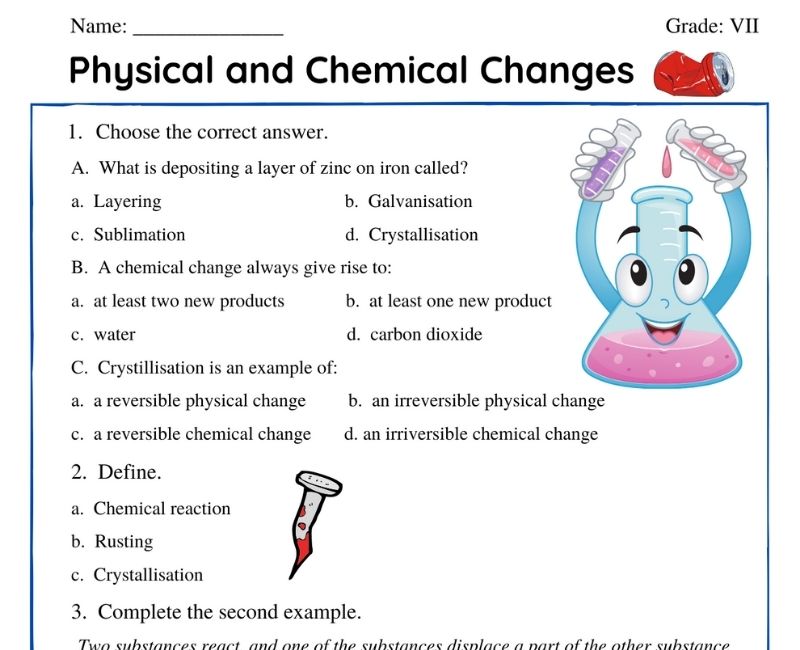
Class 7 Physical and chemical changes Worksheet
Embark on an intriguing journey into the world of matter with Class 7 Science Chapter 5, where the mesmerizing dance between Physical and Chemical Changes unfolds. This chapter offers young minds a fascinating look at how substances around us constantly transform, leading to the marvelous diversity of materials we see and use every day. The allure of this chapter lies in its ability to transform abstract scientific concepts into relatable, observable phenomena, making the learning experience both engaging and enriching.
Dive deeper with the meticulously crafted Physical and Chemical Changes Class 7 Worksheet, a treasure trove of challenges and puzzles waiting to be solved. These worksheets serve as a bridge, connecting theoretical knowledge to the practical, tangible world. Each carefully designed activity invites students to apply what they've learned in innovative ways, fostering a sense of curiosity and wonder. Through a blend of thought-provoking questions, hands-on experiments, and critical thinking exercises, these resources are crafted to spark a lifelong passion for science.
Whether grappling with the mysterious conditions that trigger chemical reactions, or unraveling the effects of physical changes on different substances, the Class 7 Chapter 5 Science worksheets make every lesson a fascinating adventure. Here, education transcends the boundaries of traditional learning, offering a vibrant playground for young explorers eager to discover the secrets of Physical and Chemical Changes.
What is chemical change class 7
A chemical change, also known as a chemical reaction, is a process where one or more substances are altered into one or more new and different substances. In a chemical change, the molecular or ionic structure of the substance is changed. Examples of chemical changes include burning of wood, rusting of iron, cooking of food, digestion of food, etc. In these changes, new substances are formed which have properties different from the original substance. For example, when wood is burned, it turns into ash, smoke and gases which are completely different from the original wood. The main characteristics of a chemical change are:
- Formation of new substances.
- Change is usually irreversible.
- Often accompanied by energy change - energy is either given out or absorbed.
- There is a change in the molecular or ionic structure of the substance.
Difference between physical and chemical change class 7
Physical Change
A physical change is a type of change in which the form of matter is altered but one substance is not transformed into another. The size or shape of matter may be changed, but no chemical reaction occurs. Physical changes are usually reversible. Examples include melting of ice, boiling of water, cutting of wood, etc.
Chemical Change
A chemical change, also known as a chemical reaction, is a process where one or more substances are altered into one or more new and different substances. In a chemical change, the molecular or ionic structure of the substance is changed. Chemical changes are usually irreversible. Examples include burning of wood, rusting of iron, cooking of food, digestion of food, etc.
Key Differences
Here are the main differences between physical and chemical changes:
- New Substance Formation: In a physical change, no new substances are formed. In a chemical change, new substances are formed.
- Reversibility: Physical changes are usually reversible, while chemical changes are usually not.
- Energy Changes: Physical changes usually do not involve changes in energy. In chemical changes, energy is either absorbed or released.
- Change in Composition: In a physical change, the composition of the substance does not change. In a chemical change, the composition of the substance changes.
Remember, the key difference between a physical and chemical change is that a physical change only alters the shape or appearance of a substance, not its chemical composition. A chemical change, on the other hand, alters the composition and properties of the original substance, forming new substances with new properties.